This document outlines the key differences in dosing response, appetite suppression mechanisms, and recommended switching protocols when transitioning from Tirzepatide to Retatrutide, based on observed user data and anecdotal reports.
1. Threshold-Dependent Clinical Response Back to Top
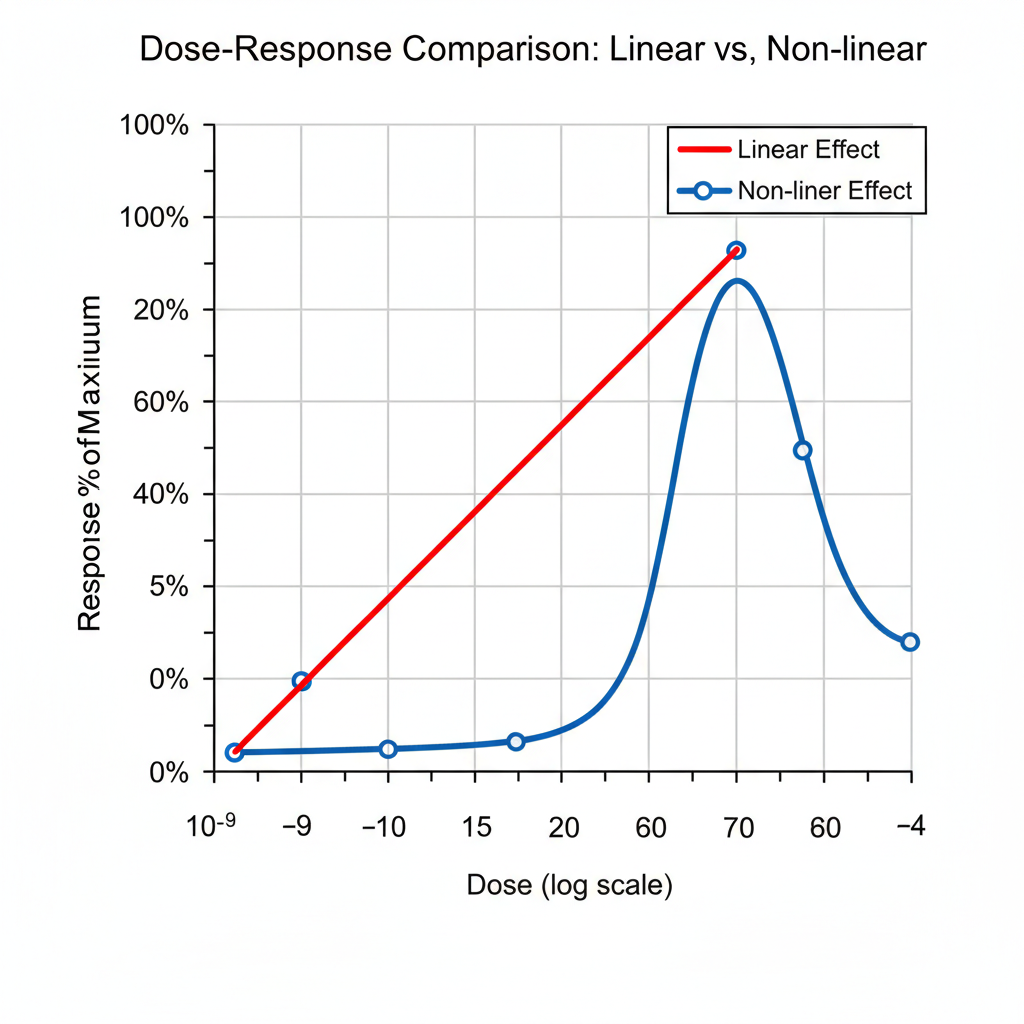
If you are coming off high-dose Tirz and moving to Reta, brace yourself for a significant difference in how the clinical effects develop. While both drugs have dose-proportional pharmacokinetics, their clinical effect profiles differ substantially.
| Compound |
Clinical Effect Profile |
Behavior |
| Tirzepatide | Gradual Escalation |
Clinical effect scales proportionally with dose increases. Appetite suppression and weight loss typically improve incrementally at each titration step. Tirzepatide has a half-life of approximately 5 days[5], supporting its weekly dosing schedule[2]. |
| Retatrutide | Threshold-Dependent |
Clinical effects emerge more dramatically at higher doses. While pharmacokinetics are dose-proportional, clinical trials show substantially greater efficacy at 8mg and 12mg compared to lower doses[7]. Retatrutide has a half-life of approximately 6 days[6]. |
2. The "Magic Zone" and Dosing Rhythm Back to Top
With Reta, the initial low doses (e.g., 3mg and 6mg) may feel completely ineffective. Clinical trials show that lower doses (e.g., 1mg) yield significantly less weight reduction compared to the 8mg/12mg doses[7]. This is a crucial point to understand: it is normal, not a failure.
- Based on observed user data and anecdotal reports, the reported "magic zone" for Reta where noticeable clinical effects truly manifest is typically between 9-12mg[3].
- It requires approximately four (4) to five (5) weekly doses at any level to achieve full physiological effect. The drug is expected to reach steady-state concentration after about four weeks[8]. This loading phase is essential for the drug's signaling cascade to be fully built and established.
- Do not chase the feeling early with extra shots. The primary mechanism relies on a 7-day rhythm of peak drug concentration followed by a drop, which is what builds and reinforces the necessary signaling over time.
Bottom line: Reta does not "climb" incrementally like Tirz; its effect tends to snap into place once adequate concentration and receptor saturation are achieved. Stick with the planned titration schedule and be patient, as the full effect often manifests near the five-week mark.
3. Comparative Hunger Control Profiles Back to Top
While both compounds are powerful appetite suppressants, they affect the psychological relationship with food differently due to their different receptor targeting profiles. Tirzepatide is a dual GIP/GLP-1 agonist[1]; Retatrutide is a triple agonist targeting GIP, GLP-1, and Glucagon receptors[1]. This triple agonism is theorized to contribute to its superior weight loss efficacy[9].
| Compound |
Hunger Control Mechanism |
| Tirzepatide |
Blunts hunger and desire. Food typically becomes a neutral sensation, often described as "meh." |
| Retatrutide |
Calms intrinsic hunger while maintaining pleasure. You still enjoy eating, but you satisfy your cravings earlier with less food ("I'm good") and do not obsess over consuming more. |
4. Stacking and Switching Protocols Back to Top
The Overrated Stack: Tirzepatide + Retatrutide
Combining the two compounds is generally considered overrated in the user community. Overlapping mechanisms of action tend to produce minimal extra clinical benefit while significantly increasing the risk of adverse effects (e.g., severe nausea) and incurring additional cost. Critically, there is no safety or testing data on the efficacy or long-term effects of stacking multiple GLP-1 agonists (e.g., Tirzepatide, Semaglutide, Retatrutide) together.
Superior Stacking Option: Cagrilintide
If you're looking to amplify the appetite suppression beyond what Reta offers, a more pharmacologically sound approach is to stack a non-GLP1 hunger suppressant. Cagrilintide, a long-acting Amylin analog[4], targets a distinct appetite pathway and offers a vastly superior option for synergy, potentially leading to greater weight loss with a known safety profile when combined with other GLP-1s like Semaglutide (CagriSema), though it remains investigational when used with Retatrutide. Based on clinical trial protocols, cagrilintide is typically initiated at 0.25mg weekly and titrated every 4 weeks (0.25mg → 0.5mg → 1.0mg → 1.7mg → 2.4mg) over 16 weeks to the maintenance dose of 2.4mg weekly. It should be administered on a different day than your GLP-1 agonist. Note: Cagrilintide remains investigational and is not FDA-approved; these protocols are derived from clinical trials and should only be followed under medical supervision.
Switching Strategy A: Overlap (Smoother Transition) - Recommended
This strategy is generally recommended for those sensitive to hunger changes or concerned about a rebound. It involves introducing Reta while Tirz is still active, utilizing a slower titration schedule. This is the recommended method as long as it is done slowly. The 98% elimination rate for Tirzepatide on max dose (15mg) is approximately 28 days[2]. After your first 4 week hold of the introductory dose, you can titrate more aggressively without having to worry. If you are currently on a dose lower than the max dose of Tirz, it's generally accepted that you don't need to be nearly as conservative unless you're worried about side effects.
- Phase 1: Overlap Start: After your last Tirz dose, wait 7 days, then start Reta at the trial's conservative introductory dose: 2mg.
- Phase 2: Conservative Titration: If well-tolerated, after the initial 28 days, Tirz is ~98% removed from your system. Titrate normally according to the trial data.
- Phase 3: Stabilization: Once you reach 9mg Reta, maintain this dose for a minimum of 8 weeks to allow full physiological effect to build before considering further increases.
- Expectations: This slower transition minimizes immediate side effects and prevents the "hunger panic" associated with a full washout, but it extends the time until you reach the Reta "Magic Zone."
Switching Strategy B: Cold Turkey (Aggressive Titration)
This method maximizes the speed of transitioning to the effective Reta dose, but carries a higher risk of immediate side effects and temporary weight rebound.
- Phase 1: Washout: Stop Tirz entirely and wait for between four (4) to five (5) weeks for the drug to clear your system[2].
- Phase 2: Reta Titration: Initiate Reta on an accelerated schedule: 3mg → 6mg → 9mg → 12mg[3], while still waiting four full weeks between each titration. (Note: This rapid dosage increase is more aggressive than the standard slow 2mg or 4mg increases recommended in clinical trials.)
- Expectations: Expect a temporary weight rebound of a few pounds during the Tirz washout phase. Weight loss typically resumes and accelerates once you reach ≥ 9mg and are five doses into the Reta protocol.
5. Split Dosing: The Pharmacological Strategy for Stability Back to Top
Split dosing is a common pharmacological technique used to mitigate the peak adverse effects (e.g., severe nausea, gastrointestinal distress) associated with certain high-concentration medications. Instead of taking the entire weekly dose at once, the total dose is split into two smaller injections (e.g., half-dose administered 3.5 days apart).
Why Consider Split Dosing?
The primary goal of split dosing is to flatten the pharmacokinetic curve—the concentration of the drug in the blood over time[10].
- Reduced Peak Concentration (Cmax): By splitting the dose, the initial "peak" concentration (Cmax) achieved immediately after injection is lower. A high Cmax is often correlated with the intensity of transient side effects like acute nausea[10].
- Higher Minimum Concentration (Cmin): By injecting the second half-dose midpoint through the week, the minimum concentration (Cmin) reached before the next weekly injection is higher. This helps maintain stable signaling, which is especially relevant for Reta's threshold-dependent behavior (Section 1).
- Maintaining Efficacy: For compounds with a relatively long half-life (like Tirzepatide and Retatrutide), splitting the dose does not diminish the overall weekly exposure (AUC - Area Under the Curve), but improves tolerance by managing the intensity of the side-effect window.
Important Caveat on Efficacy:
While two half-doses equate to the same total weekly dose, the body's pharmacological response to the flattened curve is different. For Reta, the high Cmax peak is necessary for the initial, strong signaling required for maximum efficacy. Therefore, split dosing should only be utilized by those seeking reprieve from mild to severe side effects and who prefer to remain at their current dose rather than titrating down. The technique is effective for side-effect reduction, but in many, if not most, situations, it may still be less effective overall for weight loss compared to a single, full weekly dose.
Application to Retatrutide
Given Reta's threshold-dependent nature, split dosing is often adopted by users who have reached the Magic Zone (9mg+) but experience significant side effects on the day after injection. The strategy aims to:
- Reduce post-injection side effects.
- Ensure the minimum effective concentration is sustained throughout the entire seven days, maintaining the powerful appetite suppression without the "hunger spike" that can occur just before the next weekly dose.
- Comparative Receptor Activation: Tirzepatide is a GIP and GLP-1 receptor agonist. Retatrutide is a triple agonist (GIP, GLP-1, and Glucagon) as documented in key clinical trial publications.
- Tirzepatide Pharmacokinetics & Elimination: FDA Prescribing Information, citing the half-life (~5 days) leading to ~98% elimination after approximately 5.6 half-lives (28 days).
- Retatrutide Efficacy Dosing Trials: Dosing regimen (e.g., 9mg, 12mg) used and reported in major clinical studies showing optimal efficacy.
- Cagrilintide Mechanism: Development of cagrilintide, a long-acting amylin analogue; structure, function, and clinical development confirmed in the Journal of Medicinal Chemistry.
- Tirzepatide Half-Life: NCBI StatPearls confirming tirzepatide has an elimination half-life of approximately 5 days, facilitating weekly dosing.
- Retatrutide Half-Life: Nature Medicine reporting that the pharmacokinetics of retatrutide are dose proportional with a half-life of approximately 6 days, enabling weekly subcutaneous administration.
- Dose-Dependent Efficacy (Reta): Phase 2 trial results demonstrating that weight loss increases significantly with dose, with 100% of participants achieving ≥ 5% weight loss at 8mg and 12mg, compared to lower percentages at 1mg.
- Steady State Achievement: PubMed review confirming that the pharmacokinetics of retatrutide are dose proportional with a mean half-life of approximately 6 days, supporting steady-state achievement after approximately four weeks.
- Triple Agonism Superiority: New England Journal of Medicine Phase 2 trial suggesting that the triple agonism profile supports more dramatic weight loss outcomes compared to dual agonists.
- Pharmacokinetic Principle (Cmax/Cmin): Principle that high Cmax often correlates with side effects and that dose adjustments aim to manage the concentration curve.